Erythropoiesis: Definition and Importance
Erythropoiesis is the biological process by which red blood cells (RBCs) are generated from hematopoietic stem cells. These cells are vital for oxygen transportation throughout the body, ensuring the survival and proper functioning of tissues. The process is tightly regulated to maintain a balance between RBC production and the physiological oxygen demands of the body.
Occurrence Across Life Stages
- Fetal Stage:
During fetal development, erythropoiesis primarily occurs in the liver and spleen. These organs act as temporary hematopoietic sites, producing RBCs to support the rapid growth and oxygen requirements of the developing fetus. - Adult Stage:
In adult animals, erythropoiesis shifts to the red bone marrow, where specialized niches provide an environment conducive to the differentiation and maturation of RBCs. This transition marks the establishment of a permanent hematopoietic site for continuous RBC production.
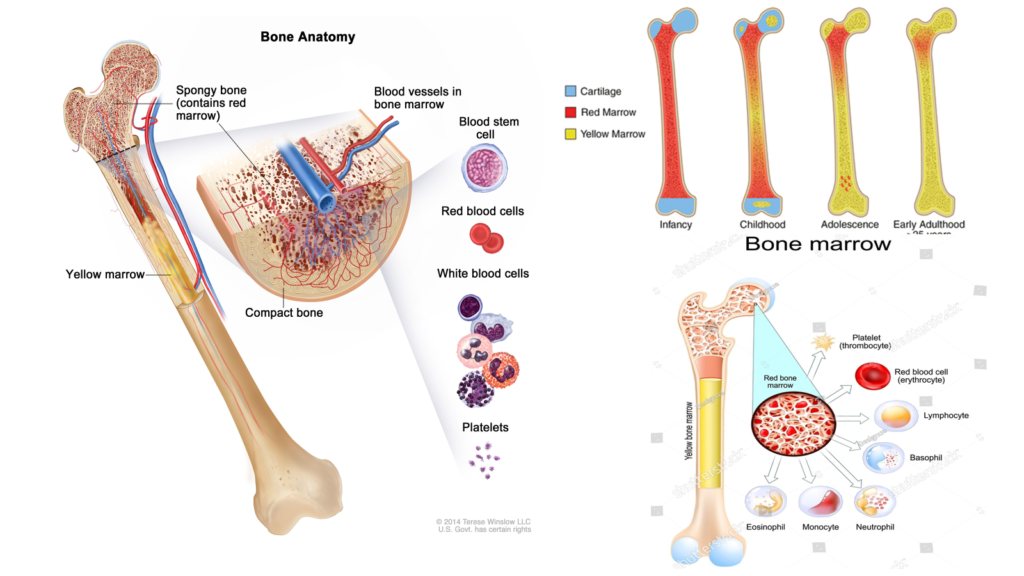
Role of Bone Marrow: Red vs. Yellow
- Red Bone Marrow:
The red bone marrow is the primary site for erythropoiesis in adults. Rich in hematopoietic stem cells, it is actively involved in producing RBCs and other blood components. - Yellow Bone Marrow:
While yellow bone marrow predominantly stores fat, under certain physiological conditions, such as severe blood loss, it can revert to red bone marrow and participate in erythropoiesis to replenish the blood supply.
Mechanism of Erythropoiesis
Erythropoiesis progresses through distinct stages, starting with pluripotent hematopoietic stem cells and culminating in the formation of mature RBCs. Key regulatory factors include erythropoietin (EPO), a hormone produced by the kidneys, which stimulates the proliferation and differentiation of erythroid progenitor cells. This finely tuned process ensures a continuous supply of functional RBCs to sustain the body’s oxygen transport needs.
By adapting to different life stages and utilizing specialized bone marrow compartments, erythropoiesis exemplifies the body’s remarkable ability to maintain homeostasis and respond to changing physiological demands.
1. Hematopoietic Stem Cells (HSCs)
- Overview: Erythropoiesis begins with multipotent hematopoietic stem cells (HSCs) located in the bone marrow. These cells have the capacity to differentiate into all types of blood cells.
- Characteristics:
- HSCs are undifferentiated and self-renewing, ensuring a sustained supply of progenitor cells.
- They reside in specialized microenvironments called “niches” within the bone marrow.
- Regulation:
- Differentiation into progenitor cells is guided by colony-stimulating factors (CSFs) and other hematopoietic growth factors.
- The fate of HSCs is influenced by signals from surrounding stromal cells, cytokines, and transcription factors.
2. Common Myeloid Progenitor (CMP)
- Transition: Under the influence of specific growth factors, HSCs differentiate into common myeloid progenitors (CMPs), which give rise to all myeloid lineages, including erythrocytes.
Erythropoietic Pathway: A Detailed Overview
Erythropoiesis is the stepwise differentiation process where hematopoietic stem cells produce mature red blood cells (RBCs). Each stage involves distinct cellular and nuclear changes, hemoglobin synthesis, and reduction in cell size. Below is a comprehensive explanation of the stages:
1. Proerythroblast
- Definition: The proerythroblast is the first committed cell in the erythroid lineage, transitioning from multipotent common myeloid progenitors (CMPs) in response to erythropoietin (EPO).
- Cell Size: Approximately 16–20 µm in diameter.
- Cytoplasm: Basophilic (deep blue) due to a high concentration of ribosomes required for hemoglobin synthesis.
- Nucleus:
- Large and centrally located.
- Fine chromatin structure, indicative of transcriptional activity.
- Key Features:
- Rapid mitotic divisions to produce a pool of erythroid precursor cells.
- Active synthesis of mRNA for hemoglobin production.
- Name Origin: “Pro-” indicates the first stage of erythroid commitment, and “-blast” signifies an immature cell capable of division and differentiation.
2. Basophilic Erythroblast
- Cell Size: 10–15 µm, slightly smaller than the proerythroblast.
- Cytoplasm: Strongly basophilic due to active ribosome synthesis.
- Nucleus:
- Reduced in size.
- Chromatin begins to condense, becoming clumped and less transcriptionally active.
- Hemoglobin Synthesis: Initiation of hemoglobin production, although ribosomal activity predominates at this stage.
- Name Origin: “Basophilic” reflects the affinity of the cytoplasm for basic dyes, owing to ribosomal RNA presence.
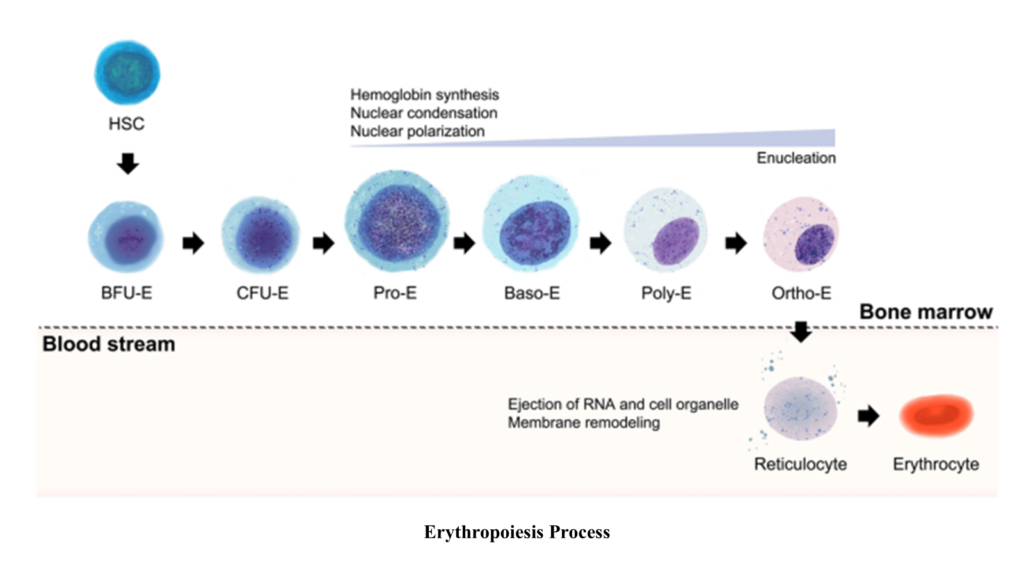
3. Polychromatophilic Erythroblast
- Cell Size: 10–12 µm, further reduced compared to earlier stages.
- Cytoplasm:
- Exhibits polychromatic staining (a mixture of blue and pink).
- Blue reflects remaining ribosomes, and pink indicates hemoglobin accumulation.
- Nucleus:
- Continues to condense.
- Chromatin becomes more densely packed, reducing transcriptional activity further.
- Hemoglobin Synthesis: Significantly increased, marking the transition to functional RBC characteristics.
- Name Origin: “Polychromatophilic” refers to the dual-staining nature of the cytoplasm, indicative of a transitional stage.
- Cell Size: 8–10 µm, nearing the size of mature RBCs.
- Cytoplasm: Eosinophilic (pink) due to hemoglobin dominance, with minimal ribosomal content remaining.
- Nucleus:
- Highly condensed (pyknotic).
- In mammals, the nucleus is ejected to maximize space for hemoglobin.
- In birds, reptiles, and amphibians, the nucleus is retained but becomes inactive.
- Hemoglobin Synthesis: Nearly complete, with the cell optimized for oxygen transport.
- Name Origin: “Orthochromatophilic” (ortho = correct, chroma = color) describes the cytoplasm’s uniform pink appearance due to hemoglobin saturation.
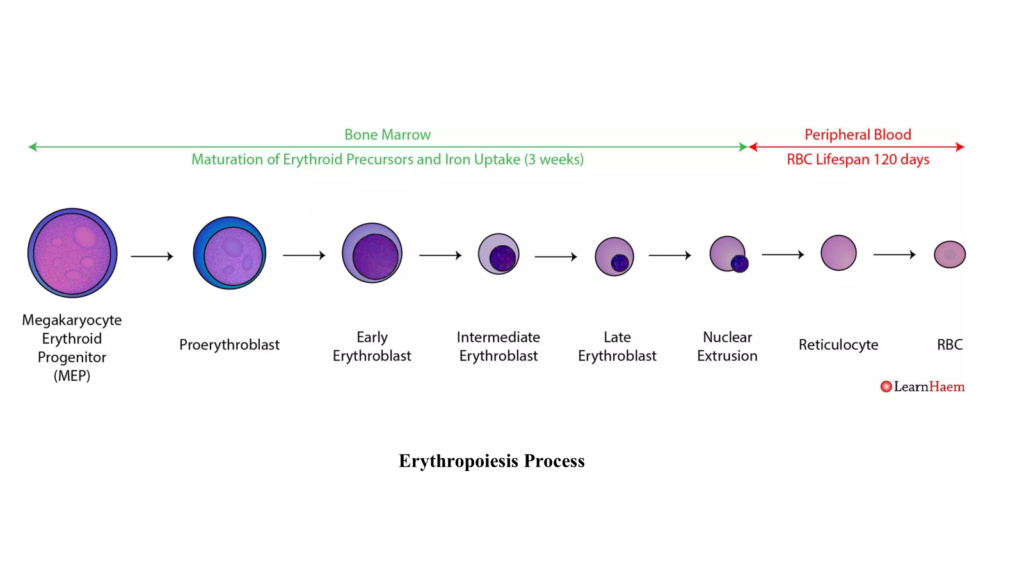
5. Reticulocyte
- Cell Size: 8–10 µm, similar to mature RBCs.
- Cytoplasm: Predominantly eosinophilic, reflecting high hemoglobin content.
- Reticulocytes retain residual organelles like ribosomes and mitochondria, visible as a reticular (network-like) structure under specific staining.
- Nucleus: Absent in mammals, allowing maximum hemoglobin storage and flexibility.
- Maturation:
- Reticulocytes are released into circulation, constituting 1–2% of total RBCs in healthy animals.
- Residual organelles are eliminated within 1–2 days, transitioning into mature erythrocytes.
- Clinical Relevance: Elevated reticulocyte counts indicate active erythropoiesis, often in response to anemia or hypoxia.
6. Mature Erythrocyte
- Cell Size: 7–8 µm, the smallest and most specialized stage.
- Cytoplasm: Fully eosinophilic due to complete hemoglobin saturation.
- Nucleus:
- Absent in mammals, increasing cytoplasmic space for hemoglobin.
- Retained in birds, reptiles, and amphibians, though inactive.
- Shape: Biconcave in mammals, enhancing surface area for gas exchange and enabling flexibility through capillaries.
- Function:
- Efficient oxygen transport from the lungs to tissues.
- Facilitates carbon dioxide removal from tissues to the lungs.
Key Terminology and Transition
- “Blast” vs. “Cyte”:
- Blast Cells: Immature, actively dividing cells (e.g., proerythroblast, basophilic erythroblast).
- Cyte Cells: Mature, non-dividing cells (e.g., reticulocyte, erythrocyte).
- Naming Reflects Function and Appearance:
- Proerythroblast: The earliest committed cell in the erythroid pathway.
- Basophilic Erythroblast: Basophilic cytoplasm due to ribosome content.
- Polychromatophilic Erythroblast: Mixed staining due to simultaneous hemoglobin synthesis and residual ribosomes.
- Orthochromatophilic Erythroblast: Uniform eosinophilic cytoplasm due to hemoglobin dominance.
Combined Table: Total Time and Stages of Erythropoiesis
| Species | Estimated Time for Erythropoiesis (Days) | Bone Marrow Stage Duration | Blood Circulation Maturation Time | Remarks |
| Cattle | 6–7 | 5–6 days | 1–2 days | Slightly longer due to slower metabolic turnover. |
| Sheep | 5–6 | 4–5 days | 1–2 days | Average duration, similar to most mammals. |
| Goats | 5–6 | 4–5 days | 1–2 days | Enhanced erythropoiesis at high altitudes. |
| Horses | 6–7 | 5–6 days | 1–2 days | Comparable to cattle due to size and metabolism. |
| Dogs | 5–6 | 4–5 days | 1–2 days | Rapid production to meet high oxygen demands. |
| Poultry | 4–5 | 3–4 days | 1 day | Shorter duration due to high metabolic rate. |
Factors Affecting Erythropoiesis in Different Animals
Several intrinsic and extrinsic factors influence erythropoiesis, leading to variations across species:
1. Oxygen Tension:
- High-altitude species (e.g., goats) show enhanced erythropoiesis due to hypoxia-induced erythropoietin production.
2. Nutritional Status:
- Deficiencies in iron, vitamin B12, folic acid, and amino acids impair erythropoiesis.
- Grazing animals rely heavily on forage quality, which varies seasonally.
3. Hormonal Regulation:
- Erythropoietin (EPO): The primary regulator of erythropoiesis, produced by the kidneys in response to hypoxia.
- Thyroid hormones and androgens stimulate erythropoiesis.
- Glucocorticoids enhance erythroid progenitor survival during stress.
4. Bone Marrow Microenvironment:
- Stromal cells in the bone marrow provide growth factors (e.g., SCF, IL-3) for erythroid cell proliferation.
5. Disease States:
- Chronic infections or inflammation reduce erythropoiesis via increased hepcidin levels, limiting iron availability (anemia of chronic disease).
- Hemoparasitic infections (e.g., Babesia in cattle) lead to hemolysis, necessitating compensatory erythropoiesis.
6. Age and Growth:
- Neonates of domestic animals exhibit rapid erythropoiesis post-birth to meet high metabolic demands.
- Aging leads to reduced marrow activity and slower erythropoiesis.
Role of Erythropoietin and Nutritional Factors
Erythropoietin (EPO)
EPO is a glycoprotein hormone primarily produced in the peritubular interstitial cells of the kidney. It plays a pivotal role in regulating erythropoiesis:
- Mechanism:
- EPO binds to erythroid progenitors via the EPO receptor (EPOR), a member of the cytokine receptor family.
- Activates intracellular signaling pathways (e.g., JAK-STAT, PI3K, and MAPK), promoting survival, proliferation, and differentiation of erythroid cells.
- Species-Specific Variations:
- In birds, EPO is produced in the kidney and liver.
- Higher EPO sensitivity is observed in high-altitude-adapted species like goats.
- Therapeutic Applications:
- Recombinant EPO is used to treat anemia associated with chronic kidney disease or chemotherapy.
Nutritional Factors
Erythropoiesis depends on the availability of essential nutrients:
- Iron:
- Required for heme synthesis.
- Deficiency leads to microcytic, hypochromic anemia, commonly seen in piglets.
- Example: Supplementation of ferrous sulfate prevents iron-deficiency anemia in intensively reared piglets.
- Vitamin B12 and Folic Acid:
- Essential for DNA synthesis in erythroid precursors.
- Deficiency leads to macrocytic anemia.
- Amino Acids:
- Necessary for globin chain synthesis.
- Protein malnutrition results in reduced erythropoiesis.
- Copper:
- Facilitates iron absorption and transport by ceruloplasmin.
- Copper deficiency, especially in ruminants, causes anemia.
Seasonal and Breed-Specific Variations
Seasonal Variations:
- Temperature and Grazing:
- Cold weather increases erythropoiesis due to higher oxygen demands for thermoregulation.
- Nutritional deficiencies during dry seasons reduce erythropoiesis in grazing animals.
- Photoperiod:
- Seasonal light exposure influences melatonin production, which indirectly affects erythropoiesis.
Breed-Specific Variations:
- Goats:
- High-altitude breeds show increased hematopoietic activity due to chronic hypoxia.
- Desert breeds like the Barbari goat exhibit adaptive erythropoiesis with higher RBC turnover.
- Horses:
- Endurance breeds (e.g., Arabian horses) maintain robust erythropoiesis for sustained oxygen delivery during exercise.
- Draft breeds show lower erythropoietic activity due to lower metabolic demands.
- Dogs:
- Sighthounds (e.g., Greyhounds) exhibit higher RBC counts and PCV, linked to their athletic requirements.
- Poultry:
- Laying hens have higher erythropoietic rates to meet metabolic demands of egg production.
- Seasonal variations in erythropoiesis are influenced by temperature and feed quality.
Research Findings on Erythropoiesis
- EPO and Hypoxia:
- A 2022 study published in Veterinary Hematology reported that high-altitude goats had 30% higher serum EPO levels compared to lowland breeds, leading to increased RBC counts and PCV.
- Iron Supplementation:
- Studies in piglets found that iron dextran injection significantly reduced neonatal anemia and improved growth performance.
- Nutritional Interventions:
- A 2023 meta-analysis revealed that supplementing vitamin B12 in ruminants improved RBC count and hemoglobin levels in 90% of studies reviewed.
- Genetic Regulation:
- Research on horses showed that genetic polymorphisms in the EPO gene are associated with variations in erythropoiesis and athletic performance.
Destruction of Red Blood Cells (RBCs): Process and Locations in Humans and Domestic Animals
The destruction of red blood cells (RBCs), known as erythrophagocytosis, primarily occurs in the reticuloendothelial system (RES), which includes the spleen, liver, and bone marrow. This process is crucial for recycling iron and other cellular components, maintaining homeostasis in the body.
In Humans:
In humans, the spleen plays the dominant role in RBC destruction. As RBCs age (typically around 120 days in humans), their membranes become less flexible and more susceptible to mechanical stress. These aged or damaged RBCs are trapped in the narrow splenic sinusoids, where macrophages engulf and break them down.
- Key Process:
- Hemoglobin is degraded into heme and globin.
- Heme is further processed into iron, which is recycled, and biliverdin, which is converted to bilirubin and excreted via bile.
- Globin chains are degraded into amino acids for reuse.
- The liver also participates by clearing defective or damaged RBCs that escape splenic destruction. Bone marrow assists in phagocytosing immature or defective erythroid cells.
In Domestic Animals:
The primary sites of RBC destruction in domestic animals are similar to humans but exhibit species-specific adaptations:
- Spleen:
- In most domestic animals (e.g., cattle, sheep, goats, and horses), the spleen serves as the principal site of RBC destruction. Its structure varies, influencing its filtration capacity. For instance, the spleen in ruminants and equines has prominent reticuloendothelial activity for filtering aged cells.
- In species like dogs and cats, the spleen is highly efficient in removing aged RBCs due to its sinusoidal structure.
- Liver:
- The liver plays a secondary role in RBC breakdown, especially during conditions where the spleen is overwhelmed or dysfunctional. Kupffer cells, the resident macrophages of the liver, engulf damaged RBCs and process hemoglobin for iron recycling and bilirubin production.
- In conditions like hemolysis (e.g., hemoparasitic infections such as Babesia in cattle), the liver’s role in erythrocyte destruction increases significantly.
- Bone Marrow:
- In some cases, erythrophagocytosis occurs in the bone marrow, where defective or immature RBCs are cleared before entering circulation. This is particularly relevant in domestic animals experiencing stress erythropoiesis.
Key Differences:
- Species Variation:
- In species like poultry, RBC destruction occurs in the spleen and liver, but the cells are nucleated, requiring additional processing compared to enucleated RBCs in mammals.
- In high-altitude species like goats, efficient RBC recycling is critical to adapt to oxygen stress.
- Physiological Conditions:
- In domestic animals, certain diseases (e.g., anaplasmosis, babesiosis) lead to premature RBC destruction, often increasing the workload on the spleen and liver.